In particular, vacuum sealing at rotating components, such as drive shafts, agitators, or mixers, poses a technical challenge that demands advanced, reliable engineering solutions. In this context, mechanical seals play a key role by ensuring separation between the internal plant environment and the outside, even under negative pressure and variable thermal cycles.
Operating principle of mechanical seals for vacuum
The operating principle of mechanical seals relies on the interaction between flat sealing faces, typically made from materials with high wear and corrosion resistance, such as silicon carbide, graphite, ceramic or stainless steel.
These faces, kept in contact by springs or compensating systems, prevent the passage of fluids or gases between two chambers, even under rotational motion.
Material selection is crucial to ensure chemical compatibility with the processed products, resistance to high temperatures (up to 250°C), and long service life, especially in sterile environments or where high containment is required.
Types of mechanical seals for pharmaceutical applications
Mechanical seals used in the pharmaceutical industry are mainly divided into two categories: lubricated and dry-running.
Lubricated mechanical seals: features and applications
Lubricated mechanical seals use a barrier liquid, typically oil or glycolated water, that circulates between the sliding faces to reduce friction, dissipate heat and prevent the ingress of contaminants.
This configuration offers longer service life under severe operating conditions and effective protection against external contamination, but it also entails greater plant complexity due to the need for pressurization, control and monitoring systems.
Moreover, in the event of barrier fluid leakage, there is a risk of product contamination, making these solutions less suitable for processes requiring very high purity standards.
Dry-running mechanical seals for sterile environments
Dry-running mechanical seals, by contrast, do not use lubricating liquids. This makes them particularly suitable for sterile environments or processes where maximum product purity is required.
The absence of liquid eliminates contamination risk and simplifies the plant setup, reducing operating costs and easing maintenance.
However, these seals are more sensitive to wear on contact faces and to temperature and pressure variations, requiring stable operating conditions and limited rotational speeds to ensure long-term performance.
In some cases, hybrid solutions can be adopted, combining dry-running sealing elements with external cooling systems to improve thermal resistance without compromising process purity.
Regulatory compliance and certifications for pharmaceutical seals
Another key aspect is compliance with industry standards such as GMP (Good Manufacturing Practices) guidelines, ATEX directives for potentially explosive atmospheres and FDA and EN 10204-3.1 certifications for materials in contact with the product.
Mechanical seals must be designed and manufactured to meet these requirements, ensuring component traceability, ease of cleaning and compatibility with validation protocols.
In particular, in API (Active Pharmaceutical Ingredients) and HAPI (Highly Active Pharmaceutical Ingredients) production, a high containment level is essential to prevent any release of harmful powders or vapors into the surrounding environment.
Advanced sealing solutions for Italvacuum vacuum dryers
With extensive experience in designing and manufacturing vacuum systems for the pharmaceutical industry, Italvacuum has adopted a targeted selection of mechanical seals to ensure maximum reliability and safety in its dryers BiEvolution, Criox, Planex , and CosmoDry.
The choice focuses on high-end solutions tailored to the sector’s specific needs, with particular attention to compatibility with sterile environments, resistance to thermal stresses, and the ability to maintain a vacuum even in the presence of rotating components.
SeccoMix single mechanical seal
The compact, robust design of dry seals ensures tightness even under deep vacuum, without using barrier liquids (process conditions from full vacuum to 6 bar(g)). This makes them particularly suitable for pharmaceutical processes where contamination prevention is paramount.
Materials such as silicon carbide and resin-impregnated graphite provide high wear and corrosion resistance, while the multiple-spring system in AISI 316 Ti ensures constant pressure between the sealing faces.
To maintain high performance over time, stable operating conditions are required, avoiding thermal shocks and vibrations that could compromise face integrity.
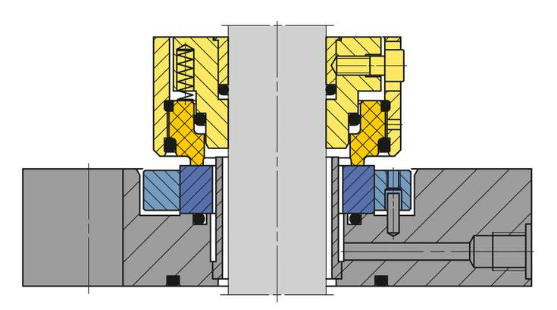
SeccoMix single mechanical seal 1 (EagleBurgmann) - Light yellow: metal support housing
- Dark yellow and dark blue: sealing rings
Dry-running system with nitrogen: double mechanical seal
An advanced configuration of the aforementioned dry seal integrates a nitrogen gas management panel.
This dry-running system feeds the seal’s barrier chamber with technical nitrogen at controlled pressure, creating an inert atmosphere that further improves vacuum tightness and reduces cross-contamination risk. Being dry and chemically neutral, nitrogen helps maintain stable conditions within the seal, protecting sliding faces from oxidation and deposits.
The control panel allows pressure regulation and real-time monitoring of deviations, ensuring safe, continuous operation even in systems subject to thermal cycles, as well as enabling planned maintenance when nitrogen flow leaking toward the process side becomes excessive.
This solution is particularly suitable for HAPI production processes, where containment and operational safety are top priorities.
Double Dry-running SeccoMix mechanical seal (EagleBurgmann) with bearing - Light yellow: metal support housing
- Dark yellow and dark blue: sealing rings

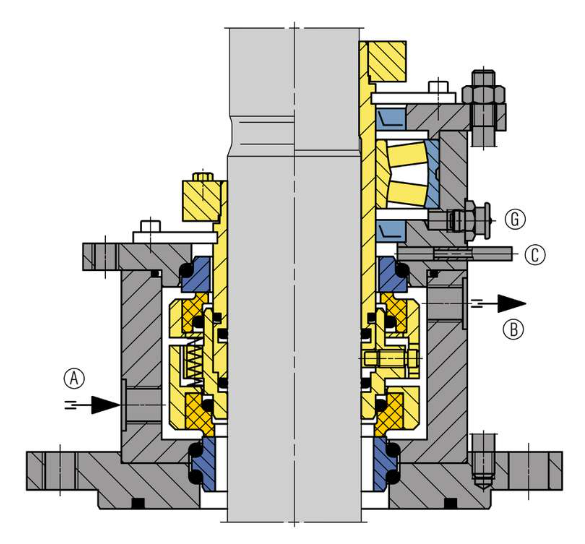
Figure 3
Planex dryer – Italvacuum patent – equipped with a double mechanical seal on the agitator shaft
Lift-Off technology: dynamic face separation
At a higher technological level and complexity, Italvacuum can implement EagleBurgmann Lift-Off mechanical seals on its vacuum dryers.
The operating principle is based on dynamic separation of the sealing faces: during start-up and shutdown, the faces remain in contact to ensure tightness, while in steady-state operation, thanks to differential pressure and the internal ring geometry, the faces slightly separate, drastically reducing friction and wear.
This behavior extends seal life, reduces energy consumption and minimizes particle generation. Lift-off technology is compatible with sterile environments and can be integrated with monitoring systems to verify separation status. Italvacuum uses this solution in high-performance systems where operational continuity and process precision are essential.
Double oil-lubricated mechanical seal
When applications require higher rotational speeds and enhanced thermal management, Italvacuum offers a double oil-lubricated mechanical seal. Thanks to an integrated lubrication system, it provides high heat dissipation capacity and longer service life under severe operating conditions (up to 220°C).
Materials such as graphite and silicon carbide ensure optimal corrosion resistance and mechanical strength at very high speeds (over 500 rpm). The secondary sealing system, consisting of FDA-certified perfluoroelastomer O-rings, ensures compatibility with process fluids and tightness under variable pressure (max. 25 bar(g)).
However, the need for continuous barrier fluid monitoring and greater system complexity requires careful management and scheduled maintenance to avoid operational issues.
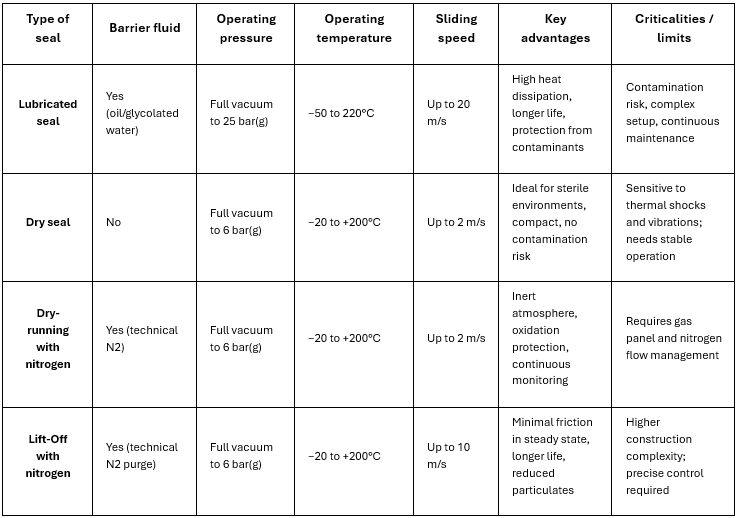
Comparison of commonly used seals on Italvacuum dryers
ATEX compliance and regulations for mechanical seals
Mechanical seals used by Italvacuum, such as EagleBurgmann SeccoMix, Dry-running, and Lift-Off, are available in configurations compliant with Directive 2014/34/EU (ATEX), suitable for use in Zone 1/21 classified areas with potentially explosive atmospheres due to pharmaceutical dusts or flammable vapors.
Their design includes antistatic materials, non-sparking separation systems, and certified components to ensure safety even without thermal monitoring.
Integration with Italvacuum systems fully meets ATEX requirements, ensuring component traceability, compatibility with GMP validation protocols, and operator protection in high-risk environments.
This is particularly relevant in API and HAPI production, where process containment and safety are priorities.
Plant integration of Italvacuum mechanical seals
From a plant engineering perspective, integrating mechanical seals into vacuum systems requires careful design that considers operating conditions, product characteristics , and cleaning and maintenance needs.
Italvacuum adopts a modular approach that allows customization according to customer specifications, ensuring maximum flexibility and compliance with industry regulations.
Integrated monitoring systems, based on pressure, temperature, and flow sensors, enable real-time detection of anomalies, reducing downtime and improving overall plant efficiency.
Italvacuum quality and reliability in vacuum mechanical seals
Italvacuum’s selection of mechanical seals reflects an engineering approach focused on quality, safety, and process sustainability.
The adopted solutions strike an optimal balance between performance, reliability, and compatibility with pharmaceutical requirements, confirming the company’s longstanding commitment, over 85 years, to technological research and continuous improvement.
 ENG
ENG ITA
ITA FRA
FRA ESP
ESP DEU
DEU